
Life Sciences Research & Development
Life sciences Research & Development is all about discovering and creating new things that help us understand and improve life, whether it’s finding new treatments for diseases, producing better crops, or understanding how living things work. It involves scientific research, experimentation, innovation and collaboration across disciplines including biology, chemistry, genetics, medicine and biotechnology
The drug discovery and development process typically involve multiple stages, each with its own roles and challenges. The pharma product development journey is intricate and complex, going through many stages from discovery to post-trade requirements.
- Discovery and Target Identification: Researchers start by identifying potential drug targets—specific molecules, proteins, or biological pathways associated with disease. Techniques such as high throughput testing and computational modelling help in this process.
- Target Validation: Once targets are identified, they must be validated to ensure that the combination of these targets will have the desired therapeutic effect. It uses cell culture models, animal studies, and genetic techniques.
- Lead Discovery and Optimization: Researchers then look for chemical compounds that interact with the target. Large compound libraries are screened, and promising ones are improved to improve efficacy, safety, and pharmacokinetics
- Preclinical Development: The preclinical development phase involves extensive testing in laboratory and animal models to identify toxicity optimization and assess safety and efficacy.
- Clinical development: Encouraging results enter clinical trials, which occur in three stages:

- Regulatory approval: Upon successful completion of clinical trials, a new drug application (NDA) is submitted to a regulatory agency such as the FDA or EMA for approval.
- Post-marketing evaluation: Once approved, the product continues to be evaluated for safety and efficacy to identify and address long-term adverse effects.
Each process requires a lot of time, resources and expertise, and the success rate is very low. However, improved drug development can dramatically improve health care and the treatment of various diseases.
Challenges and Veeva’s Role in the Solution
The process involves various challenges such as data governance, compliance and communication among different parties and teams. This is where Veeva Vault comes into play. Veeva offers an integrated, cloud-based system that simplifies document management, ensures compliance, and enhances collaboration, maximizing efficiency and reducing the risk of errors throughout the pharmaceutical lifecycle.
Why Veeva Vault?
Traditionally, companies had to use applications for content and separate applications to manage associated data. Veeva Vault is the only content management platform with unique capabilities to manage content and data. Companies can now eliminate system, location, and country silos and streamline end-to-end processes across marketing, medical, clinical, regulatory, quality and safety.
What is Veeva Vault?
Veeva Vault platform is a Cloud-based Software for the Global Life Sciences Industry and suite of applications. Vault provides organizations with a “single source of truth” to store their documents, data records, and manage business practices in a single system.
A single source of truth is the practice of bringing all business data (and documents) to one location. This allows all data records (and documents) to be accessible in one centralized location.
Founded: Veeva Systems was founded in 2007.
Mission: To help life science companies bring their products to market faster and more efficiently.
Veeva Features
- Veeva Vault: Integrated suite of content management applications.
- Veeva CRM: Broad customer relationship management tailored for life sciences.
- Veeva Network: Cloud-based master data management solution.
- Veeva Data Cloud: Provides comprehensive data for commercial and clinical use.
Veeva Vault in Action
Veeva Vault is used by life science companies to quickly and securely manage their data and access it in one central location. It helps them streamline processes, comply with regulatory standards, and improve efficiency.
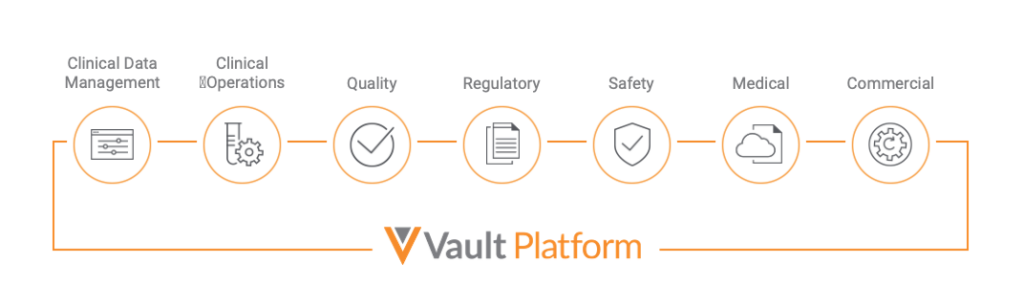
For example, a pharmaceutical company might use Vault to store and manage important documentation related to manufacturing and marketing. Vault also provides tools to track progress and automate processes. By using Vault, a company can ensure that its data remains secure and organized and that its systems remain compliant.
Veeva Vault Modules
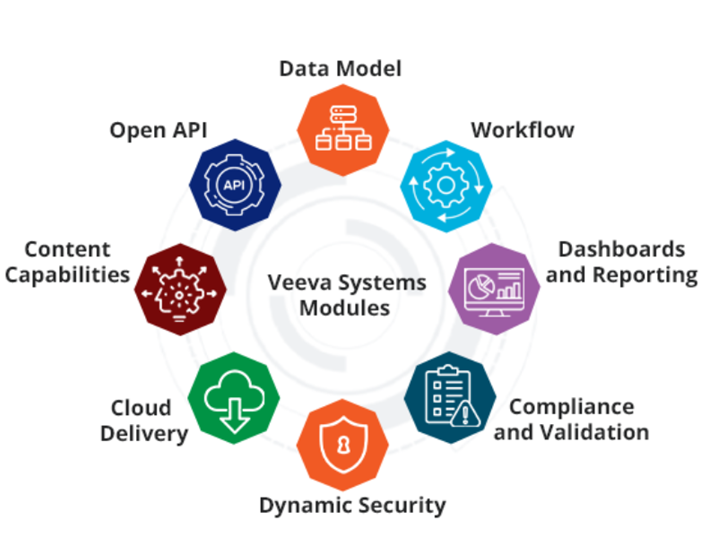
- Data Model: With the Vault Object Framework (VOF) we can easily create custom objects to manage business processes that require structured data..
- Workflow: Automate and track business processes with configurable workflow. Provides assignment, routing, email notifications, escalation, and tracking of work items.
- Dashboards and Reports: Quickly gain data insights with integrated reporting and dashboards to boost faster, informed decision-making. Easily share customizable reports, charts, and dashboards – allowing users to summarize and explore both structured and unstructured data
- Compliance and Validation: Ensures compliance with regulatory standards and maintains system validation for quality.
- Dynamic Security: Provides flexible and robust security measures to protect sensitive information.
- Cloud Delivery: Provides scalable and reliable cloud-based solutions for global access.
- Content capabilities: Manage documents, video, and images with rich capabilities including versioning, lifecycle, annotation, rendering, electronic signatures, watermarking, document generation, templates, and share Rule-based configuration that supports complex business needs for compliance, collaboration, and records management.
- Open API: Easily integrate with other systems, migrate data, or automate processing using the comprehensive REST APIs.
Veeva Vault Benefits
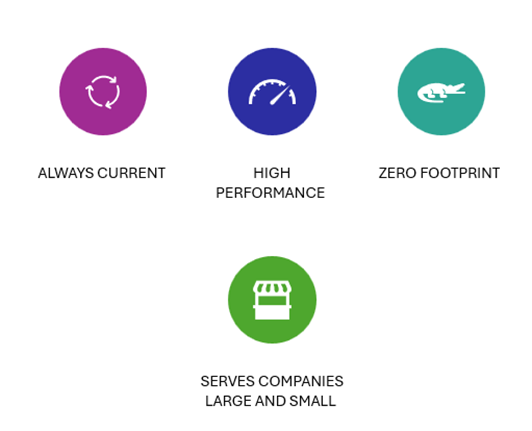
- Always current: Veeva is constantly innovating and delivering new IQ/OQ validated releases, so you’ll never be stuck with outdated software.
- High performance: Performance is constantly monitored and optimized to ensure all applications have high performance.
- Zero footprint: No software to install, no hardware to maintain, or expensive modifications.
- Serves companies large and small: Subscriptions go up or down to accommodate any size of business.
Veeva Vault – Case Study
Bay Area Life Sciences organization leverages RIM & SafetyDocs for cost effective & scalable resourcing model to reduce operational overheads. Read the complete story here.
Conclusion
Veeva Vault is a game-changer for the pharmaceutical industry, offering a comprehensive solution to streamline processes and ensure compliance. Ready to transform your R&D operations? Explore our Veeva Vault Support Services to maximize your efficiency and innovation. Contact us today to learn how we can help you leverage the full potential of Veeva Vault.


