The Drug Development and Research field is evolving rapidly making it more challenging to safeguard patients and monitor the adverse effects of drugs, or adverse drug reactions (ADRs). Every year, drug safety cases increase by 10-15% due to more complex drugs, better-informed healthcare professionals and patients, and an increase in patients’ health issues.
Life sciences companies face a challenge—they need to cut costs related to drug safety, enhance process efficiency, hasten the time-to-market for new drugs, and monitor adverse events effectively during post-market surveillance. In this digital-first age, where the volume of data on such events is enormous and regulatory reporting demands continue to grow, it’s crucial to rethink current pharmacovigilance processes and embrace a digital-first strategy through intelligent automation and advanced analytics solutions.
Life sciences companies are producing more specialty drugs, which require monitoring for adverse effects in more places and through more channels. This increases manual compliance costs, removing resources from valuable growth-supporting activities such as safety monitoring and risk reduction.
The significant increase in adverse event reports to the US Food & Drug Administration—up by 84% from 2014 to 2020—highlights an urgent need for more robust pharmacovigilance systems. This surge is a statistical concern and a clarion call for enhanced safety measures. This explains why industry analysts anticipate a compound annual growth rate of 11.5% in global pharmacovigilance spending through 2028.
As a result, life sciences companies are adopting automation in pharmacovigilance. This strategic change intends to deal with adverse events faster, lower operational costs, enhance patient safety, and generate scalable growth opportunities.
By moving from only responding to risks to actively predicting and avoiding them, these companies are transforming how safety is managed in the digital age. They are using advanced technology to simplify and improve the pharmacovigilance automation process.
What is Pharmacovigilance?
Pharmacovigilance is the science and activities related to the detection, assessment, understanding, and prevention of adverse effects or any other drug-related problems. Its primary goal is to improve patient safety and ensure that the benefits of a medication outweigh its risks.
Need for Pharmacovigilance Automation
The need for pharmacovigilance automation stems from several key factors:
- Growing volume of healthcare data
- Evolving regulatory compliance
- Manual processes are time-consuming and prone to errors
- Need for data integration from different sources
Overall, pharmacovigilance automation helps improve the effectiveness of monitoring systems, ensuring that patient safety is maximized and regulatory requirements are met efficiently.
Life sciences companies want to automate pharmacovigilance (PV) for these reasons:
- to handle adverse events faster
- lower costs
- enhance patient safety, and
- create growth opportunities with compliance at scale.
They want to go from responding to risks to anticipating and avoiding adverse events while automating routine tasks such as data entry.
Using AI for Pharmacovigilance Automation
In response to this, and their full conversion from a reactive to a proactive avoidance of risks, life science companies begin to take over the role of AI in automating the pharmacovigilance process. This approach, more forward-looking in nature, not only smoothens routine tasks like data entry but materially improves the entire pharmacovigilance workflow.
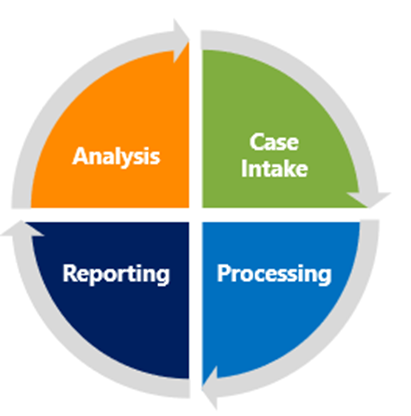
The Pharmacovigilance process can be divided into four steps, starting with Case Intake. This first step is essential as it underpins how the adverse events are captured, managed, and prioritized. AI empowers companies to deal with large amounts of data at pace and with accuracy, thereby making each subsequent step of the entire pharmacovigilance process, from initial intake to final reporting and analysis— more efficient, responsive, and proactive in safeguarding patient safety.
Massive changes continue to occur in how ADRs are reported and managed in the developing field of pharmacovigilance. With advances in digital technology, the prospects for ADR reporting expanded to include not only its traditional methods of reporting, like FDA reports and phone calls, but also online forms, e-mails from physicians, and social media.
Information flow is thus a critical need for the operation of a practical case triage system, filtering and prioritizing information in such a manner that allows for the best possible usage of resources.
The identified cases undergo a detailed and ethical case processing process. Every ADR is coded with standardized terminologies, such as MedDRA, ensuring the integrity and completeness of data. An ICSR is developed, and a complete narrative is compiled, including the description of the adverse event, timeline, medical history, and other critical details. Such a narrative is instrumental for further signal detection or assessment.
The process of a case continues but goes on to be submitted, scrutinized for the risk involved, and tracked systematically. With this top-down approach, every reported adverse event is critically reviewed for patterns and potential risks, bringing value through information toward informed decision-making on drug safety.
The Takeaway
Organizations must consider key questions such as the level of advancement in pharmacovigilance automation, the realistic outcomes and return on investment of these technologies, and the challenges that must be addressed to achieve optimal results.
- How advanced is the automation process in the PV function right now?
- Realistic outcome to expect from process automation with clear ROI.
- What are the organizational challenges and the required actions to achieve the ideal outcome?
- How can we assess and find immediate benefits to convince leadership that automation efforts are successful?
- What long-lasting automation projects can be planned with realistic roadmaps and timelines?
WinWire’s Pharmacovigilance Case Intake Automation Framework – Improving Operational Efficiency
Our primary objective is straightforward—enhance patient safety by boosting operational efficiency. At WinWire, we focus on improving pharmacovigilance processes using top-notch technology. Our PV Case Intake Offering makes everything from collecting to analyzing and reporting data more accessible, which helps us work more efficiently and meet safety standards.
We use AI and machine learning to streamline tasks like data entry, sorting cases, and analyzing them. This allows teams to concentrate more on Signal Analysis and Risk-benefit assessment.
| Benefits | Reduction |
| PV case processing cost | 40-60% |
| Case in-take and triage time | 40-50% |
| Effectiveness, quality of compliance, and patient safety | Boosted |
Pharmacovigilance Case Intake Process Automation: A Case Study
Recently, our client, a global leader in animal health, aimed to improve the efficiency of the pharmacovigilance process for the drugs they produce and distribute in different markets. The WinWire solution’s ability to perform pharmacovigilance automation in multiple languages helped unify and simplify the ADE intake and triage process, significantly reducing total processing time.
Subsequently, using the WinWire solution, they were able to reduce the time they needed in the ‘Case Intake’ process, improving the efficiency of their operations and increasing their ability to respond to drug safety issues.
What’s Next?
Interested in improving your PV processes and prioritizing patient safety? Reach out to WinWire to learn more about how our pharmacovigilance automation solutions can help streamline your operations.


