Customer Success Story
Bay Area Life Sciences organization leverages regulatory information management to reduce submission time and maximize revenue.
READ STORYWith ever-changing national regulations, data standards, and complexities around preparing for regulatory submissions, regulatory affairs organizations in Healthcare and Life Sciences industry are convinced and driven towards smarter processes and intelligent systems to optimize costs, maximize accuracy and minimize timelines for the submission.

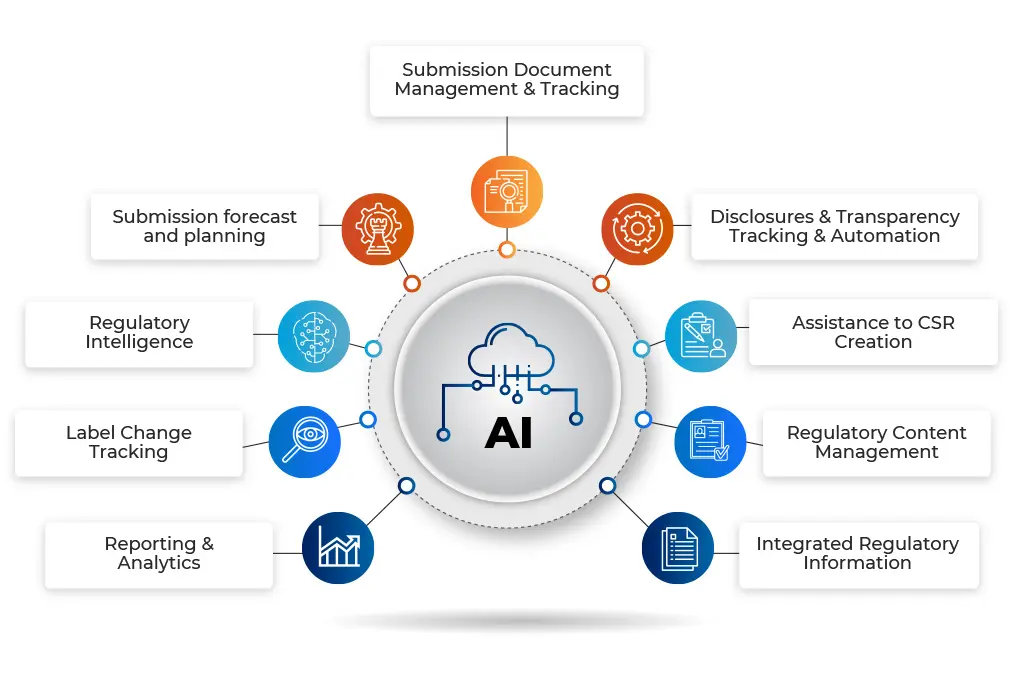
RIMTrack – is a cloud-ready Artificial Intelligence (AI)-based new age Regulatory Information Management System.
Developed from scratch, its mission is to help organizations prepare submissions accurately and efficiently, as well as streamline regulatory processes related to tracking, licensing, approvals, regulatory and competitive intelligence, clinical trials, and reporting across global sites and stakeholders.
RIMTrack is designed to seamlessly integrate with various existing systems with data security at its core.
Integrate with existing RIM system and achieve complete end-to-end management of regulatory life cycle process.
Stay ahead of regulatory compliances by enhancing speed and accuracy of the submissions process.
Eliminate or cut-down all your legacy systems and streamline entire regulatory information tracking
Bay Area Life Sciences organization leverages regulatory information management to reduce submission time and maximize revenue.
READ STORYLet’s discuss how we can build intelligent, purpose-driven AI experiences to transform your business.