Clean Rooms are said to provide a conducive environment (for research, development and manufacturing of equipment or processes) with a low level of environmental pollutants such as dust, airborne microbes, aerosol particles, and chemical vapors.
With the growing emphasis on quality checks & controls, zero tolerance on defects and stringent manufacturing practices, the demand of clean rooms has increased manifold. The Clean rooms are now being designed as specially constructed enclosed areas, environmentally controlled with respect to airborne particles, temperature, humidity, air pressure flow patterns, air motion, vibration, noise and viable organisms.
Major application areas of clean rooms are in the electronic industry, pharmaceuticals, aerospace, automotive, information technology and many others requiring more stringent manufacturing conditions. The Clean Room applications range from the fabrication of microscopically small sub-assemblies, electronic devices and instruments, to the increasing demand for more sterility and purity in drugs, foods and a germ-free atmosphere for medical and biological applications.
Contaminants can come from a wide variety of sources: air (the cleanliness of which is defined according to the classes of clean rooms and classes of dust), water, chemicals, the physical plant itself, and personnel.
Examples of high humidity in Semiconductor and Pharmaceutical Clean Rooms
Microcircuits and microchips manufacturing requires hygroscopic polymers called photo resists to mask circuit lines for etching process. Because of their hygroscopic nature, they absorb moisture and, therefore, microscopic circuit lines are cut or bridged, resulting in circuit failures. In addition, in semiconductor manufacturing, when the humidity level fluctuates in a wafer fabrication area, a multitude of problems can occur. Bake-out times typically increase, and the entire process generally becomes harder to control. Humidity levels above 35% RH make the components vulnerable to corrosion. Additionally, as developer solvents are sprayed onto the wafer, the solvents evaporate rapidly, cooling the wafer enough to condense moisture from the air. This extra water can change the developer characteristics and be absorbed onto the semiconductor layers. This can cause swelling and further product quality problems, necessitating additional process control.
In Pharmaceutical manufacturing facilities, high humidity causes fine powders to absorb moisture, clogging the powder feed to the tableting press. Powder inconsistency caused by moisture absorption results in crumbling tablets and clogged tablet dies. Variation in humidity means difficult adjustments in bed temperature and spraying rates, resulting in heat damage and moisture intrusion. Also, humidity in air duct-work creates moist places for bacterial colonies to grow and causes process contamination.
Relative Humidity in Clean Rooms should be controlled between 35-40% RH (this is a function of the process requirement) for year-round operation. These RH levels are generally maintained in a narrow band ±2 % RH at temperatures below 20°C (70°F).
Bry-Air Desiccant Dehumidifiers can maintain dew points at very low levels consistently, such as (-) 60°C, a manifold reduction in the air moisture, way beyond what can be achieved by a standard HVAC grade refrigeration system.
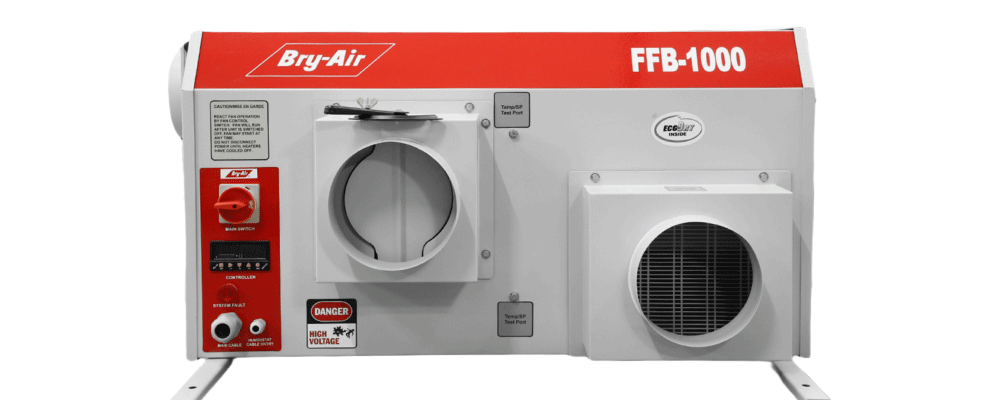
The powder coated, CNC fabricated compact desiccant dehumidifiers (FFB series 170-4500 CMH) are lightweight and can be installed above the false ceiling.

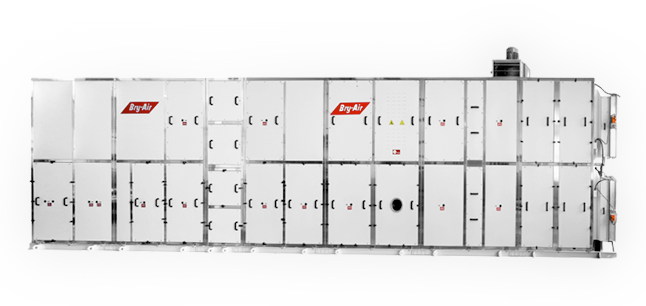
For complete air handling and environment control needs (ranging from 2,500 CMH to 25,000 CMH)

Uncontrolled humidity can lead to circuit failures, corrosion, and product quality issues in semiconductor and pharmaceutical clean rooms.




